Abstract

Background: Chemotherapy resistance and subsequent relapse remain a major cause of childhood cancer mortality, particularly for infants (<12 months of age) with KMT2A-rearranged (R) B-acute lymphoblastic leukemia (B-ALL), who have extremely poor clinical outcomes with event-free survival <20% for highest-risk patients. KMT2A (MLL1) is a histone lysine N-methyltransferase involved in HOX-family and MEIS1 gene regulation that is commonly rearranged with other gene partners to form oncogenic KMT2A fusion proteins that drive acute lymphoid or myeloid leukemogenesis. Recent preclinical and early clinical studies have reported successful targeting of the menin scaffold protein within KMT2A fusion complexes in adults with KMT2A-R acute leukemias (Wang ASH 2020 abstract #115, Stein ASH 2021 abstract #699), but minimal data in pediatric patients exist to date. We hypothesized that the selective menin inhibitor ziftomenib (KO-539) would have potent activity in preclinical patient-derived xenograft (PDX) models of infant and non-infant pediatric KMT2A-R ALL and could enhance chemosensitivity in these often-chemoresistant leukemias.
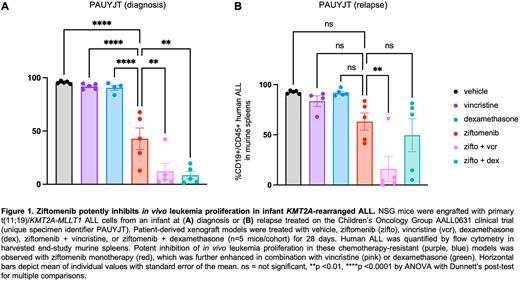
Methods: To test these hypotheses, we first measured the ability of ziftomenib to inhibit in vitro viability and KMT2A-menin complex protein targets in human KMT2A-R and KMT2A wild-type ALL cell lines in vitro using Cell Titer Glo and immunoblotting assays, respectively. We then assessed the ability of ziftomenib as monotherapy or in combination with chemotherapy to inhibit in vivo leukemia proliferation in PDX models of de novo or relapsed KMT2A-R ALL harboring various fusion partners (n=8; Loftus Haematologica 2021) after drug dose-finding in a bioluminescent KMT2A-R ALL cell line xenograft model. NSG mice engrafted with human KMT2A-R ALL cells were randomized to treatment with vehicle, ziftomenib 150 mg/kg via oral gavage (PO) daily x 5 days/week, vincristine 1 mg/kg intraperitoneally once weekly, dexamethasone 1 mg/kg PO daily x 5 days/week, ziftomenib + vincristine, or ziftomenib + vincristine for 3-6 weeks depending on rate of ALL progression in control animals necessitating study termination. Human CD45+ CD19+ ALL cell counts were measured weekly via retro-orbital venous bleeding and in end-study murine spleens via quantitative flow cytometry analysis as previously described (Tasian Blood 2017a, Hurtz JCI 2020).
Results: We observed preferential in vitro sensitivity to ziftomenib of KMT2A-R ALL cell lines HB11;19 (half-maximal inhibitory concentration [IC50] 11.1 nM), KOPN-8 (IC50 49.1 nM), and SEM (IC50 32.6 nM) versus KMT2A wild-type NALM-6 cells (IC50 400.8 nM). Ziftomenib treatment of infant, non-infant pediatric, and young adult KMT2A-R ALL PDX models (comprised of more common KMT2A-AFF1 and KMT2A-MLLT1 rearrangements or other less common KMT2A fusions) induced significant inhibition of in vivo leukemia proliferation compared to vehicle treatment. Despite the remarkable potency of single-agent ziftomenib in all models tested, combination of ziftomenib with vincristine or dexamethasone further enhanced reduction of human ALL disease burden in end-study murine spleens, even in aggressive relapsed infant ALL PDX models (representative data in Figure 1).
Conclusions: Our data demonstrate in vitro target inhibition and remarkable in vivo activity of the menin inhibitor ziftomenib against infant, pediatric, and young adult KMT2A-R ALL. Ziftomenib monotherapy activity was further augmented in most models when combined with conventional chemotherapy drugs vincristine or dexamethasone, which were well-tolerated in PDX mice. Based upon early clinical safety/tolerability data for ziftomenib in adult patients with acute leukemias and our pediatric-specific data described herein, a phase 1 clinical trial of ziftomenib in combination with multi-agent chemotherapy for children with relapsed/refractory KMT2A-rearranged, NUP98-rearranged, or NPM1-mutant acute leukemias is planned.
Disclosures
Kessler:Kura Oncology: Current Employment, Current equity holder in publicly-traded company. Tomkinson:Kura Oncology: Current Employment. Burrows:Kura Oncology: Current Employment, Current equity holder in publicly-traded company. Tasian:Aleta Biotherapeutics: Consultancy; Syndax Pharmaceuticals: Consultancy; bluebird bio: Consultancy; Beam Therapeutics: Research Funding; Kura Oncology: Consultancy, Research Funding; Incyte Corporation: Research Funding; GSK: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal